Regulatory Pharmacist
Lifestrong Marketing Inc.
İş tanımı
Avantajlar
Devletin Zorunlu Sağladığı Faydalar
13. Ay Ödemesi, Pag-Ibig Fonu, Philhealth, SSS/GSIS
Diğerleri
Şirket Sosyal Etkinlikleri
Avantajların Avantajları
Çalışan İndirimi
İzin ve İzin
Ölüm İzni, Doğum Günü İzni, Hükümetin Zorunlu İzin, Hastalık İzni, Tatil İzni
- Prepares and check submission of License to Operate renewal and other inter-related regulatory correspondence with FDA.
- Timely monitoring of product, license, permit and other licenses for renewal and ensure that all FDA license, certificates, and permits are updated and valid.
- Reviews Standard Operating Procedure based on the actual activity.
- Ensures that all pertinent documents for Cosmetic, Food, Drug and Medical Device are available for FDA inspection.
- Compiles and maintain complete technical data of each product by timely update of LTO’s master file.
- Ensures reliable IPO/Trademark monitoring
- Assists in addressing all customer inquiries by providing retention and product test results within 2 working days upon request.
- To ensure that product samples of new batches are distributed for testing 7 working days upon receipt.
- To provide product testing to Purchasing Dept. 20 working days after product test distribution or before product release to the market (for urgent testing).
- Ensures that formulation conforms to FDA standards and is within the approved limit.
- Ensures that labels of new and existing items conform to FDA Standards.
- Checking of e-Portal for newly approved CPN/CPR’s. Download, file and update the Notifications Summary.
- Generates barcode and monitoring of registration on-line.
- Ensures that Vidamed warehouses are complying with FDA requirements.
- Assists FDA inspector with the needs during inspection and makes sure that company is compliant.
- Conducts product tests for all finished goods distributed and imported by the company.
- Maintain current knowledge of relevant regulations in Cosmetic/Food/Medical device/Drugs and keeping up to date with changes in regulatory legislation and guidelines.
- Assists in updating related departments and regulatory head on latest regulatory requirements and FDA advisories.
- Coordinates the schedule of pest control.
- Monitoring in conducting annual audits of all active suppliers.
- Coordination with manufacturers for Product Information File for filing based on ASEAN Cosmetic Directive.
- Conducts a Product Information File for filing based on ASEAN Cosmetic Directive.
- Processing of initial and renewal of agreements and request for updated GMP and ISO of all active suppliers.
- Perform other related duties as required by FDA and other tasks assigned as needed.
Angel Joy Cauilan
Talent Acquisition Associate Lifestrong Marketing Inc.
Çalışma konumu
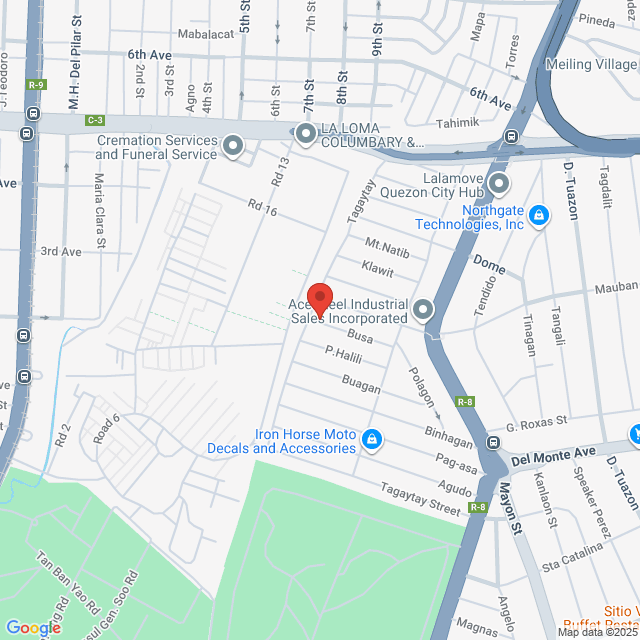
98 Busa, Caloocan, 1404 Metro Manila, Philippines
Yayınlandı 22 October 2025
Benzer İşler
Daha fazla görRegulatory Pharmacist
 Main Source Manufacturing and Trading Corp.
Main Source Manufacturing and Trading Corp.₺14.5-18.2K[Aylık]
Tesis içi - Caloocan1-3 Yıl TecrübeÜniversite mezunuTam zamanlı

MAIN SOURCE MANUFACTURING AND TRADING CORPHR Manager
Regulatory Affairs Pharmacist Manager - 5 yrs Experience
 Dempsey Resource Management Inc.
Dempsey Resource Management Inc.₺29.1-32.7K[Aylık]
Tesis içi - Caloocan5-10 Yrs ExpÜniversite mezunuTam zamanlı
Aisa Refugia
Regulatory Affairs
 Global Quest Consulting Group
Global Quest Consulting GroupPazarlıklı
Tesis içi - San Juan1-3 Yıl TecrübeÜniversite mezunuTam zamanlı

Avie VinalonRecruiter
Regulatory Pharmacist
 Kareila Management Corporation
Kareila Management Corporation₺18.2-21.8K[Aylık]
Tesis içi - Makati1-3 Yıl TecrübeÜniversite mezunuTam zamanlı

Cris Quinzanos,CRSPTalent Acquisition Specialist
Inventory Pharmacist
 Variance Trading Corporation
Variance Trading Corporation₺14.5-16.7K[Aylık]
Tesis içi - Quezon City1-3 Yıl TecrübeÜniversite mezunuTam zamanlı

georgierosas vtchrHR Recruitment

Lifestrong Marketing Inc.
StratejiFinanse edilmemiş
101-500 Çalışan
Tüketici ürünleri
İşe alınan iş ilanını görüntüle
Bossjob'a katılın
Bossjob Güvenlik Hatırlatması
Eğer pozisyon yurt dışında çalışmanızı gerektiriyorsa lütfen dikkatli olun ve dolandırıcılığa karşı dikkatli olun.
İş arayışınız sırasında aşağıdaki davranışlara sahip bir işverenle karşılaşırsanız, lütfen hemen bildirin
- kimliğinizi saklıyor,
- bir garanti vermenizi veya mülkünüzü tahsil etmenizi gerektiriyorsa,
- sizi yatırım yapmaya veya fon toplamaya zorluyorsa,
- Yasadışı menfaatler topluyor,
- veya diğer yasa dışı durumlar.
